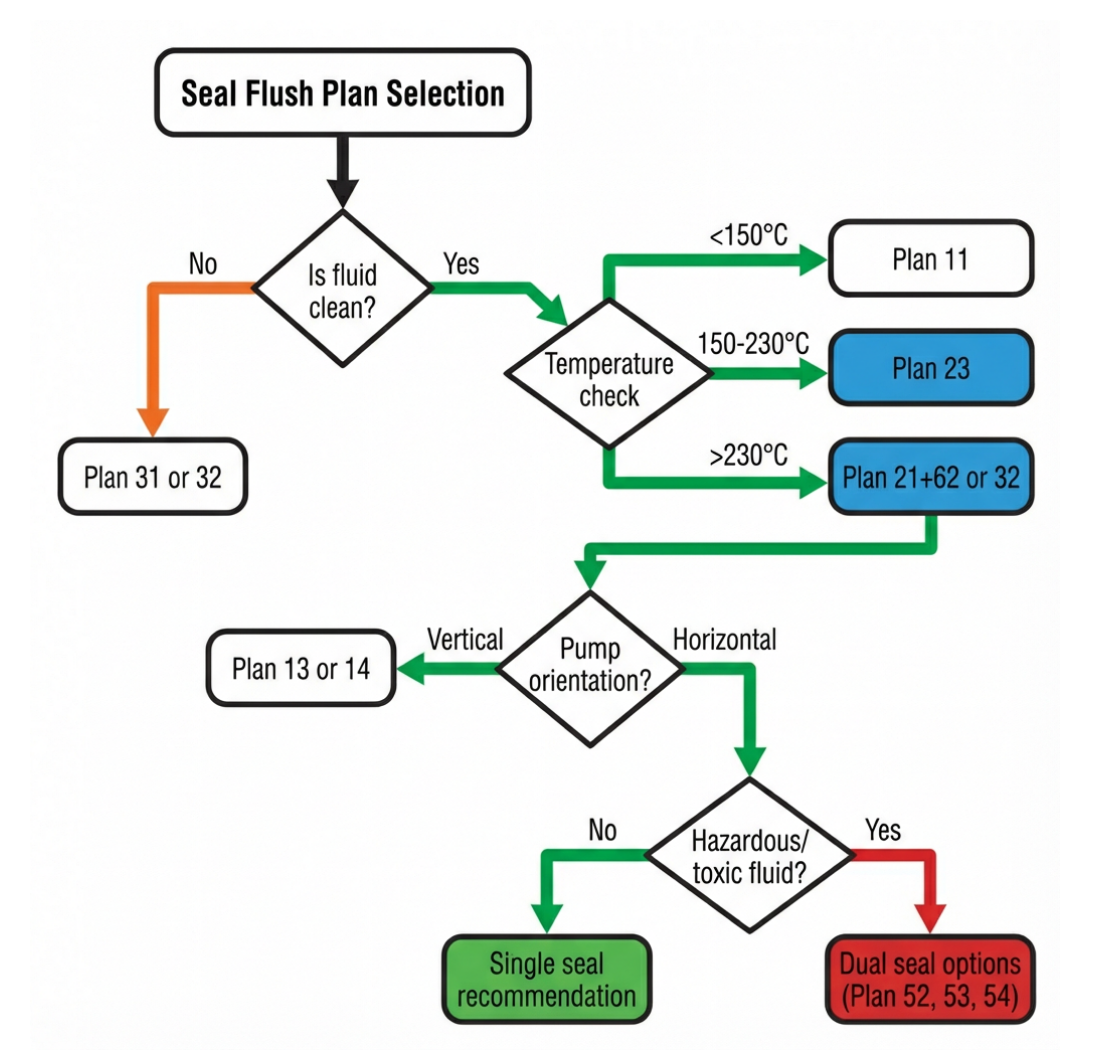
Mechanical seals are the number one cause of pump failure in pharmaceutical applications. According to GEA, “special attention must be paid to the shaft seal of the pump, as it is the number one cause of failure.” Yet many pharmaceutical plants operate with sealing technology 25 years out of date, replacing failed seals without addressing the compliance gaps that caused the failure.
This guide covers the specific FDA, USP, and ASME BPE requirements that apply to pharmaceutical pump seals. Most compliance resources focus on valves, fittings, or heat exchangers. Pump seals present unique challenges because they’re dynamic seals with rotating components exposed to both process fluid and CIP/SIP cycles.
FDA and USP Standards for Pharmaceutical Pump Seals
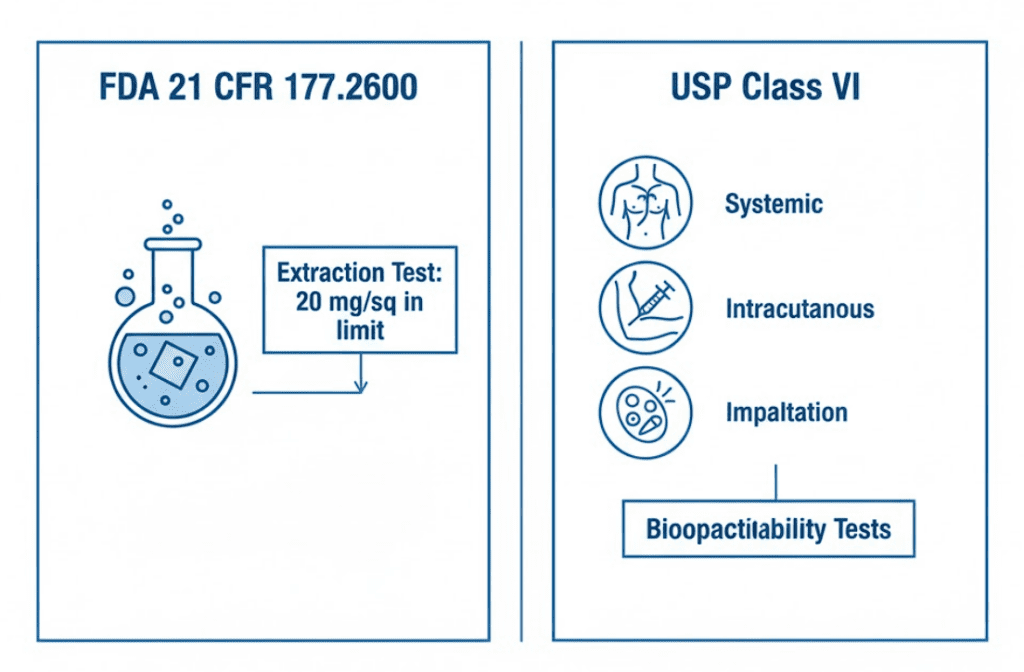
Pharmaceutical pump seals must meet two distinct regulatory frameworks: FDA 21 CFR 177.2600 for material safety and USP Class VI for biocompatibility. Many procurement teams assume these are interchangeable. They’re not.
FDA 21 CFR 177.2600 governs rubber articles intended for repeated use with food and pharmaceutical contact. The standard sets extraction limits: no more than 20 mg per square inch for aqueous extraction (7-hour water reflux) and less than 20 mg per square inch for fatty extraction (7-hour hexane reflux). FDA compliance means the material passed these extraction tests. It does not mean the finished seal is “FDA certified”—that certification doesn’t exist.
USP Class VI establishes biocompatibility requirements through invasive animal testing absent from FDA protocols. USP Class VI requires three tests: systemic injection test, intracutaneous test, and implantation test. This testing evaluates whether the material causes toxic reactions when in contact with biological systems. For pharmaceutical applications involving injectable drugs or sterile manufacturing, USP Class VI is far more rigorous than FDA food-contact standards.
For GMP compliance requirements in pharmaceutical pump seals, you need both standards. FDA compliance ensures the elastomer won’t leach harmful substances into your product. USP Class VI ensures the material won’t cause adverse biological reactions. A seal meeting only FDA requirements may be acceptable for water systems but inadequate for API manufacturing.
ASME BPE Surface Finish Requirements
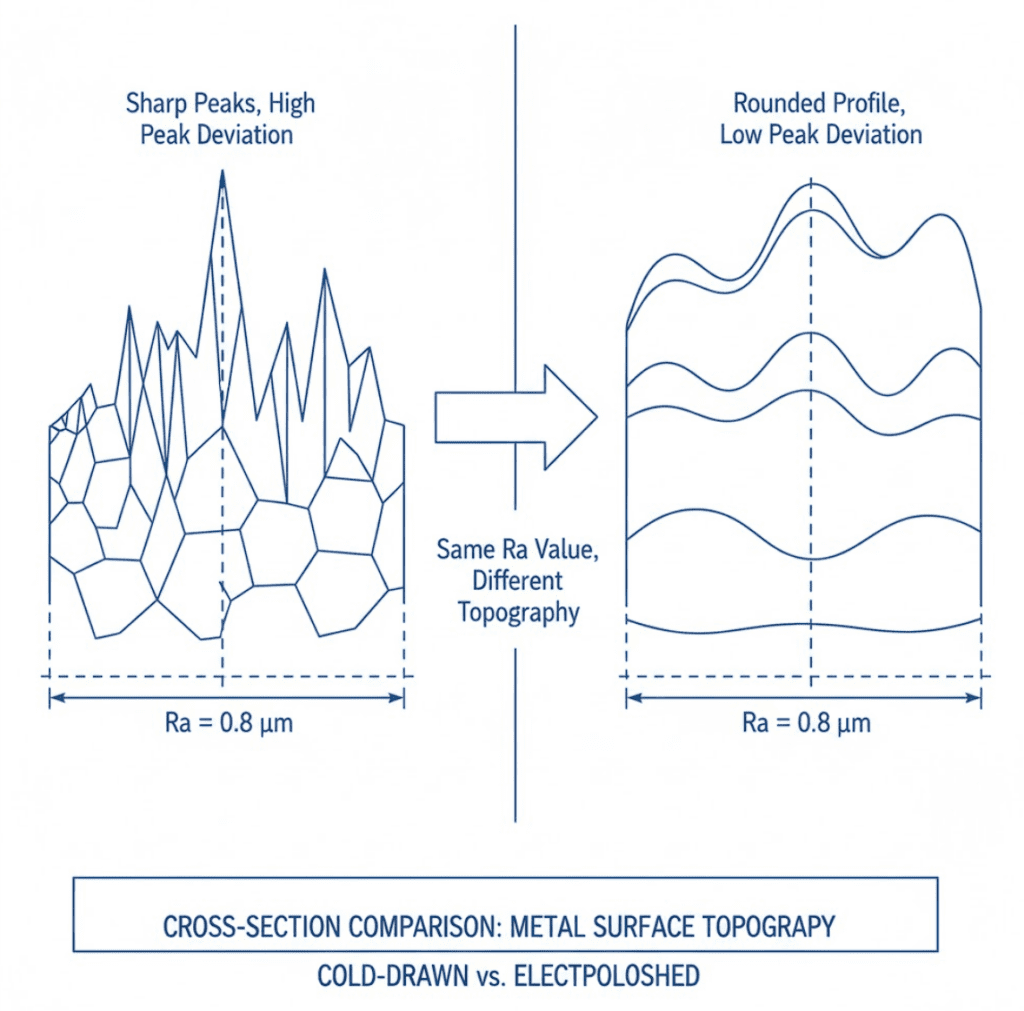
ASME BPE defines surface finish classifications from SF0 (no requirement) through SF6 for bioprocessing equipment. For pharmaceutical pump seals, SF4 is the most commonly specified: a maximum roughness of 0.38 micrometers (15 microinch) Ra with electropolishing required.
The most common mistake I see is specifying surface finish by Ra value alone. Ra measures average roughness, but two surfaces with identical Ra values can have very different topography. As Alfa Laval points out, a cold-drawn surface at 0.12 micrometers Ra had maximum peak deviation almost three times higher than an electropolished surface at 0.32 micrometers Ra. The electropolished surface actually performed better for cleanability despite the higher Ra number.
When to specify SF4:
- Sterile processing and aseptic manufacturing
- API manufacturing with direct drug contact
- High-purity water systems (WFI, purified water)
- Applications where bacterial adhesion is critical
When SF1-SF3 may be acceptable:
- Buffer preparation systems
- Utility water applications
- Non-sterile bulk processing
- Where validated cleaning procedures demonstrate adequate microbial control
The decision isn’t SF4 versus cheaper alternatives. The decision is matching surface finish to your specific contamination risk. Over-specifying wastes money. Under-specifying creates compliance gaps. Base your specification on process validation requirements and risk assessment, not blanket industry assumptions.
Compliant Seal Materials for Pharmaceutical Pumps
For pharmaceutical water pumping, the industry standard is silicon carbide seal faces paired with EPDM elastomers. This combination handles CIP (Clean-in-Place) and SIP (Steam-in-Place) cycles while meeting FDA and USP requirements.
Seal Face Materials:
Silicon carbide (SiC) faces dominate pharmaceutical pump applications because of their hardness, chemical resistance, and thermal conductivity. However, silicon carbide can corrode in Water for Injection (WFI) applications. The extremely low conductivity of WFI creates electrochemical corrosion conditions that cause unpredictable seal failures. If you’re experiencing unexplained short seal life in WFI pumps, investigate SiC material grade before replacing with the same specification.
Carbon-graphite faces paired against SiC provide a softer running combination suitable for clean services. For abrasive or highly corrosive applications, SiC versus SiC offers maximum wear resistance but requires better lubrication.
Elastomer Selection:
Elastomer choice for secondary seals depends on your temperature requirements and chemical exposure:
| Elastomer | FDA/USP Available | Max CIP Temp | Max SIP Temp | Best For |
|---|---|---|---|---|
| EPDM | Yes | 150C | 200C (oxygen-free) | Water, steam, mild chemicals |
| FKM (Viton) | Yes | 200C | Limited | Oils, solvents, acids |
| FFKM (Kalrez) | Yes | 230C+ | 327C (specialized) | Aggressive chemicals, highest temps |
| Silicone | Yes | 200C | Limited | Clean room, low-pressure |
EPDM handles most pharmaceutical water and steam applications. For aggressive cleaning agents or higher temperatures, FFKM provides the broadest chemical resistance but at much higher cost. The mistake is specifying FFKM everywhere “just to be safe” when EPDM would perform identically at a fraction of the cost.
Dynamic pump seals face different stresses than static gaskets. The rotating seal face experiences frictional heat, pressure cycling during CIP/SIP, and potential dry-running during startup. These conditions demand materials qualified for dynamic service, not just static chemical compatibility.

Documentation and Supplier Verification
Before you start procurement, make sure you have a clear documentation specification. Contractor disputes over seal compliance often stem from vague original specifications that leave accountability unclear.
Required Documentation Checklist:
Request these documents from your seal supplier:
- Material Certificates (EN 10204 3.1 minimum)
- A 3.1 certificate contains actual test results from the specific material lot, endorsed by the manufacturer’s quality department
- A 3.2 certificate adds third-party verification
- For pharmaceutical applications, 3.1 is typically the minimum; 3.2 may be required for critical applications
- FDA 21 CFR 177.2600 Compliance Letter
- Statement that elastomers meet extraction requirements
- Specific material grades tested, not generic “FDA compliant” claims
- USP Class VI Certification
- Test reports showing systemic, intracutaneous, and implantation results
- Certification should reference specific material grade and production lot
- Surface Finish Certification
- Ra measurement with instrument calibration documentation
- Electropolishing certification if SF4 or higher specified
- Lot Traceability
- Ability to trace seal components to raw material batches
- Critical for sanitary seal standards compliance
Red Flags:
- Supplier cannot provide material-specific certifications (only generic compliance statements)
- No lot traceability system
- Surface finish stated without measurement method
- USP Class VI claimed but only FDA documentation available
- Certifications reference different material grades than supplied parts
If a supplier hesitates to provide complete documentation, that hesitation tells you something about their quality system.
Compliance Verification Checklist
Before specifying pharmaceutical pump seals, verify these requirements:
Standards Compliance:
- [ ] FDA 21 CFR 177.2600 compliance confirmed for all elastomers
- [ ] USP Class VI certification obtained for drug-contact applications
- [ ] ASME BPE surface finish classification specified (SF4 for sterile, SF1-3 acceptable for non-sterile with risk assessment)
Material Selection:
- [ ] Seal face material compatible with process fluid (watch SiC in WFI)
- [ ] Elastomer temperature rating covers CIP/SIP requirements
- [ ] Dynamic seal performance verified, not just static compatibility
Documentation:
- [ ] EN 10204 3.1 or 3.2 material certificates requested
- [ ] Lot traceability confirmed
- [ ] Supplier compliance letters reference specific material grades
Properly specified pharmaceutical pump seals with modern materials can achieve mean time between failures of five years or more, compared to one year with outdated specifications. The investment in proper compliance documentation pays for itself in reduced unplanned downtime and audit confidence.